Abstract
Introduction
Rituximab (R) administration results in significant outcome improvement in B cell precursor acute lymphoblastic leukemia (B-ALL) patients (pts), but is usually restricted to pts with ≥20% CD20+ leukemic blasts. Yet, this arbitrary cut-off is not proven biologically sensible. Moreover, CD20 expression might differ between blood (pb) and bone marrow (bm) and varies under prednisone during early treatment. In the present GMALL08/2013 trial R is administered to all BCR-ABL1-negative B-ALL pts irrespective of the initial leukemic CD20 expression. We assessed the initial and post-prephase CD20 expression in GMALL08/2013 pts and correlated the values with MRD response after Induction I (Ind I) and Consolidation I (Cons I). A historical B-ALL GMALL07/2003 cohort without R treatment and with available CD20 expression and MRD data was used to evaluate for R-unrelated effects.
Methods
Comparative immunophenotypic quantification of CD20 expression in 207 B-ALL pts was performed at diagnosis (pb d0 and/or bm d0) and/or (a/o) after a 5-day dexamethasone- and cyclophosphamide-containing prephase (pb d6) under EuroFlow standardized procedures. CD20 median fluorescence intensities (CD20-MFI) and percentages of CD20+ B-ALL blasts/all blasts (%CD20+ BL) were assessed.
Minimal residual disease (MRD) was determined after Ind I (after 1x R) and Cons I (after 4x R) by quantitative PCR for clone-specific immune gene rearrangements to stratify pts as molecular complete response (MolCR, MRD negativity, assay sensitivity at least 1x10 -4), molecular intermediate response (MolIR, MRD positive non-quantifiable or positive <1x10 -4) and molecular failure (MolFAIL, MRD ≥1x10 -4).
Results
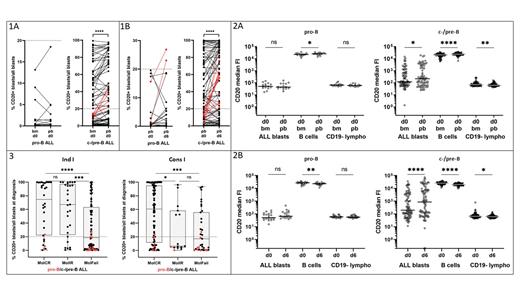
bm d0/pb d0. In 91 paired bm d0/pb d0 samples %CD20+ BL as well as the CD20-MFI were significantly higher in pb in common/pre-B ALL (c/pre-B ALL) (n=76: paired t-test: p<.0001 and p<.0001) and in normal mature B cells (CD20-MFI paired t-test; pro-B/ c/pre-B p=0.026/p<.0001), but not in pro-B ALL (n=15: paired t-test: p=.81 and p=.76) (Fig. 1A, 2A). Notably, in 6/76 (7.9%) c/pre-B ALL pts the %CD20+ BL in bm d0 was lower and in corresponding pb d0 higher than the arbitrary cut-off of 20%.
pb d0/pb d6. CD20 expression of circulating blasts significantly increased after a 5-day prephase in c/pre-B ALL but not in pro-B ALL in 106 paired pb d0/pb d6 samples (paired t-test of CD20-MFI and %CD20+ BL; n=20 pro-B ALL, p=.09 and p=.25; n=86 c/pre-B ALL, p<.0001 and p<.0001). Normal mature B cells presented with the opposite effect (CD20-MFI paired t-test; pro-B/ c/pre-B: p=0.005/p<.0001) (Fig. 1B, 2B). Notably, the %CD20+ BL in pb d0 was lower and in corresponding pb d6 higher than the arbitrary cut-off of 20% in 2/20 (10.0%) pro-B ALL and 12/86 (13.9%) c/pre-B ALL pts.
Molecular response under R. The values of %CD20+ BL were correlated with MRD response after Ind I and Cons I (Fig. 3). Since the CD20 expression in the present cohort was shown to be significantly modulated in a drug- and compartment-dependent manner, we used the highest measured value of %CD20+ BL out of the three available values per patient (bm d0, pb d0 a/o pb d6). In the historical cohort (n=145) one value per patient for %CD20+ BL was available.
Due to low CD20 expression pro-B ALL did not show any differences in the %CD20+ BL among the risk groups in the present (Ind I n=23, Cons I n=20) and the historical (Ind I n=11; Cons I n=11) cohort. The differences in %CD20+ BL in relation to molecular response were significant in c/pre-B ALL between MolCR and MolFAIL after Ind I (n=127) and Cons I (n=120) (Mann-Whitney test: p=.0002 and p=.0028) and of lower significance between MolIR and MolFAIL after Ind I (p=.013) and between MolCR and MolIR after Cons I (p=.029) in the present cohort. Within the historical cohort (Ind I n=145, Cons I n=143) no significant differences were observed.
Conclusions
Leukemic CD20 expression was modulated between compartments (bm d0/pb d0) and showed a significant increase in a drug-dependent manner in c/pre-B ALL (pb d0/pb d6) probably in response to dexamethasone. The results might challenge the conventional eligibility criteria for CD20 targeted treatment in c/pre-B ALL.
MRD persisters showed lower initial CD20 expression compared to MRD responders in the present cohort consistently receiving R, but not in the historical cohort without R treatment. Accordingly, R seems to improve the early MRD response predominantly in pts with higher CD20 expression.
Supported by DJCLS
Szczepanowski: Amgen: Speakers Bureau. Trautmann: Amgen: Speakers Bureau. Ritgen: Roche: Consultancy, Other: Travel support, Research Funding; Abbvie: Consultancy, Other: Travel support, Research Funding; Chugai: Consultancy; MSD: Consultancy, Other: Travel support; Celgene: Other: Travel support. Nachtkamp: Celgene: Other: Travel Support; bsh medical: Speakers Bureau; Jazz: Speakers Bureau. Viardot: Kite/Gilead: Honoraria, Membership on an entity's Board of Directors or advisory committees; Amgen: Membership on an entity's Board of Directors or advisory committees; Bristol-Myers Squibb: Honoraria, Membership on an entity's Board of Directors or advisory committees; F. Hoffmann-La Roche Ltd: Honoraria, Membership on an entity's Board of Directors or advisory committees; Novartis: Honoraria, Membership on an entity's Board of Directors or advisory committees; University Hospital of Ulm: Current Employment. Baldus: Jazz: Honoraria; Celgene/BMS: Honoraria; Amgen: Honoraria; Novartis: Honoraria. Schwartz: Morphosys: Research Funding; Gilead: Other: Travel grants, Speakers Bureau; Pfizer: Honoraria, Speakers Bureau; Amgen: Membership on an entity's Board of Directors or advisory committees, Other: Travel grants, Speakers Bureau; Novartis: Speakers Bureau; Jazz Pharmaceuticals: Other: Travel grants, Speakers Bureau; BTG International Inc: Membership on an entity's Board of Directors or advisory committees; MSD Sharp & Dohme: Membership on an entity's Board of Directors or advisory committees; Basilea: Other: Travel grants. Goekbuget: Pfizer: Consultancy, Other: Research funding for institution; Amgen: Consultancy, Other: Invited talks for company sponsored symposia (with honoraria); Research funding for institution; Astra Zeneca: Other: Invited talk for company sponsored symposia (with honor); Gilead/Kite: Consultancy; Novartis: Consultancy, Other: Research funding for Institution; Jazz Pharmaceuticals: Other: Research funding for institution; Incyte: Other: Research funding for Institution; Cellestia: Consultancy; Erytech: Consultancy; Morphosys: Consultancy; Servier: Consultancy, Other; Abbvie: Other. Brüggemann: Amgen: Other: Advisory Board, Travel support, Research Funding, Speakers Bureau; Incyte: Other: Advisory Board; Janssen: Speakers Bureau.
Rituximab administration to patients with CD20-negative (<20% CD20+ blasts/all blasts) BCR-ABL-negative B-ALL.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal